Common vaccine handling practices can have an effect on beef carcass quality. Most livestock producers are now aware of the potential for tissue damage, lesions or other carcass defects that can occur when vaccines are administered.
That awareness has been the impetus for moving injection sites away from valuable muscle cuts to the injection site triangle (see Figure 1) in the neck.
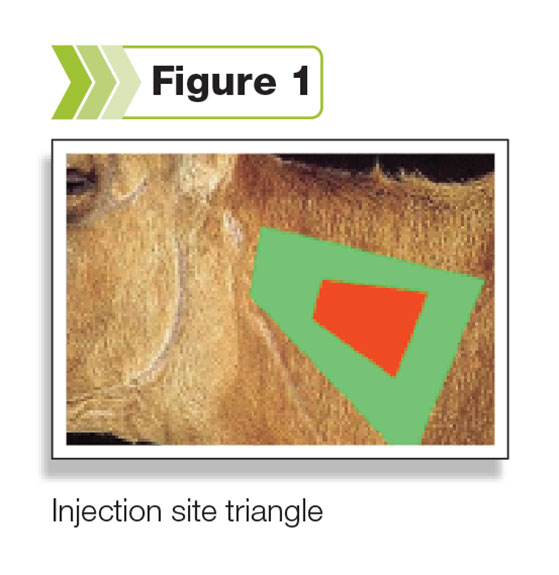
This practice alone reduces the amount of trim or cutout required at the processor. There are, however, additional concerns about vaccine handling other than just injection sites.
Vaccines need to be stored, handled and administered properly to ensure maximum vaccine efficacy.
When vaccine efficacy is reduced, there is an increased risk of disease or sickness, which can result in an increase in the use of antibiotics.
Additional use of injectable antibiotics elevates the potential for carcass quality defects.
Improving vaccine efficacy
Vaccine efficacy can be influenced by several factors, including general herd health or condition, administration of vaccines within label parameters and vaccine handling practices.
Vaccines should be administered to healthy animals. Sick or excessively thin animals often fail to mount a sufficient immune response to a vaccination to produce any lasting immunity to the disease pathogen.
Animals whose diets are deficient in certain minerals can also fail to respond to vaccination.
Following the administration parameters on the label is essential. For example, a vaccine to prevent calf scours, when administered to the cow prior to calving, must be given within a specific window of time to be effective.
If given too early or too late, it will have minimal, if any, effect on the targeted organism.
Additionally, the dosage rate and route of administration as indicated on the product label are critical to product efficacy in the animal.
Several handling-related practices can impact vaccine efficacy. These include transport and storage temperatures, syringe care, product mixing and exposure to ultraviolet rays.
Most vaccine labels recommend storage from 35 to 45ºF. Killed vaccines that are frozen, even for a short period of time, become useless.
Excessive warming also reduces vaccine efficacy. This is important at all stages (transport, storage and chuteside).
Use a min/max thermometer to help ensure proper storage temperature.
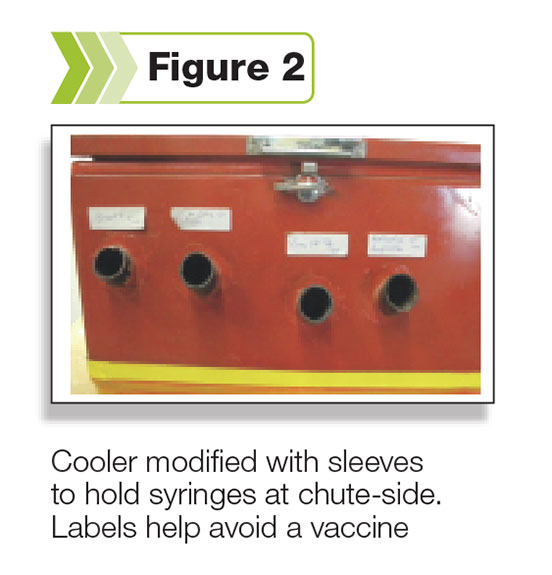
Use of a cooler when chuteside is recommended (see Figure 2), which will also help protect against damage caused by ultraviolet rays (sun).
Proper syringe care is important. Syringes should be cleaned after use. Internal parts should be cleaned with hot water only.
Soaps and disinfectants can leave a residue that will inactivate vaccines.
Multi-dose syringes should be disassembled for cleaning.
Be sure to completely dry the cleaned syringe and then store in a clean, dry place.
There are two areas of concern when it comes to product mixing. First, modified-live vaccines should not be mixed until needed.
Due to the nature of the vaccine, modified-live vaccines start to deteriorate as soon as they are mixed. Never mix more than can be used in one to two hours.
Modified-live vaccines are ineffective if not used within this time frame. Use of a transfer needed to mix modified-live vaccines is recommended.
When administering vaccines, never mix two different vaccines together, whether in the bottle or in the animal by giving injections too close together.
The bottom line
Handling, storing and administering vaccine properly is critical to vaccine efficacy and reducing vaccine-related carcass quality defects.
When livestock producers follow these recommended practices, they will help ensure maximum immune response to the vaccine and should experience fewer herd health problems.






