This might shock you, but we’d have to go back in the herbaria record 226 years to say with any degree of certainty that the shrub-steppe and grasslands of the U.S. and British Columbia were untouched by the invasive non-native winter annual grass Bromus tectorum.
Why? Because, unbeknownst to those who said “go West, young man, and grow up with the country” and made it a reality, cheatgrass was insidiously hitchhiking into the vast grasslands of North America by the end of the 17th century.
By 1890, when steam engines began hauling grain threshers around the country, farmers were beginning to take notice of the odd-looking awned grass clinging to the thresher reel. Many probably thought it was a godsend; cattle do eat it, after all. If only we had known that by 1920, with the invention of diesel-powered combines, the stage was set for cheatgrass to invade every nook and cranny of the arid grasslands of the West.
During the economic depression and drought of the 1930s, it became obvious to ranchers, researchers and policy-makers that the trifecta of a great drought, a great depression and a great defoliation of native forbs had allowed the fire-feeding cheatgrass to dominate millions and millions of acres in what now seems like the blink of an eye.
Taking that 200 years of introduction into perspective, you can appreciate the excitement when USDA Agricultural Research Service (ARS) researchers in Pullman, Washington, after 32 years of exhaustive research and investigation, isolated a naturally occurring strain of the soil bacteria Pseudomonas fluorescens that produces an inhibitory compound that can stop cheatgrass in its tracks.
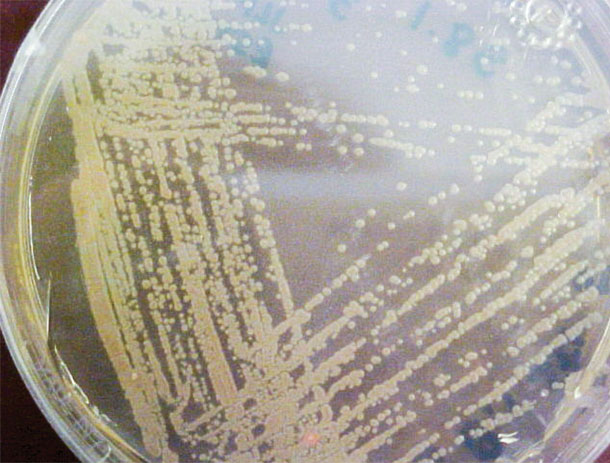
“We screened 25,000 strains of bacteria to isolate 12 strains of P. fluorescens collected from the Washington-Idaho region that inhibit the roots of cheatgrass,” says Dr. Anne Kennedy, lead researcher on the project at the USDA-ARS Pullman laboratory.
To register the naturally occurring soil bacteria as a biological herbicide, though, has taken the better part of 30 years. Weed-suppressive bacteria work by decreasing the weed seed bank and suppressing seedling vigor.
“We have to screen each strain to make sure it does not inhibit a desired species,” like wheat and native grasses, she says. Screenings also have to be done to ensure that aquatic plants, invertebrates and species on up the food chain are not harmed by the bacterium.
The USDA-ARS is applying for EPA registration, and the application is in review. Once that is accomplished, the USDA-ARS will license the bacteria to a company to manufacture the bacterium in bulk. Of course, next came the hurdles and setbacks associated with launching a new biocide product.
But now, Kennedy says, “We have an experimental use permit to conduct plot work from states all over the western U.S. We are allowed to plant 10 acres of land per target isolate per year. In the first year, we only see 20 to 40 percent suppression, but by year three, in all plots, we are seeing that 50 percent of the cheatgrass is gone.”
The results in wheat fields are even more encouraging. “What we see in wheat fields is: Once the bacteria get working, the cheatgrass is reduced and the wheat is healthier and much quicker to green up,” Kennedy says.

The cheatgrass will still flower in the treated plots, but its root growth is significantly inhibited and produces less tillers. The result is a much smaller plant that is only 2 to 3 inches tall instead of an 18-inch-tall plant. “The impaired plant has this distinctive dirty green color and only a single stem,” Kennedy notes.
Licensees of the product will need a bio-herbicide facility large enough to produce massive amounts of the product that would be needed for retail sale. The good news is: Their research has shown that only a pint of actively growing bacterium Pseudomonas fluorescens applied in the fall is needed per acre to begin to suppress cheatgrass and see it disappear over three to five years as other plant species become more competitive.
So how is this bacteria applied? Kennedy says late-fall applications when air temperatures are cool (below 50ºF) and rains are prevalent have the highest success. This allows for the weed-suppressive bacteria to establish in the fall so that when spring weed growth occurs, the bacteria populations are high enough to suppress the cheatgrass.
To date, spring applications have not been consistent in weed suppression, and weed-suppressive bacteria will not reduce the growth of standing weed plants.

The weed-suppressive bacteria can be applied to frozen soils or snow, especially if more snow is in the forecast. Bacteria survival is poor if air temperatures are too high or the bacteria are exposed to sunlight on the soil surface for too long.
Rain or snow is needed to mobilize the bacteria from the surface into the soil. Limiting factors of application in late-season cold temperatures are freezing spray nozzles and hoses.
State herbicide application licenses are needed to apply these bio-herbicides. Kennedy says after application, the bacteria will be active in the soil for four to six years.
Another important factor to consider when fighting cheatgrass, and optimizing the effectiveness of weed-suppressive bacteria, is to have native or desired plant species present. Kennedy says application of bacteria alone without some desirable plants present will result in only a short-lived reduction of cheatgrass.
If no native plants are present in the seed bank, drill-seeding of desirable grasses may be necessary. Ideally, reseeding desired plants should follow application of the bacteria in the same year or in the fall of the next year after bacterial application.
In areas where desirable plant seeding is used, grazing should be delayed until seeded plants have a chance to establish a sufficient root system. Additional monitoring should continue on weed-suppressive bacterial areas to address any cheatgrass re-infestation if native plant populations do not increase.
Kennedy also mentions that abandoned farmland poses perhaps the greatest challenge to restoring native rangeland. These sites often contain few native seeds, and past disturbance has resulted in reduced organic matter or eroded soil.
Other sites that pose great challenges are soils that have produced long-term monoculture crops. She notes that more time, tillage, herbicide spray passes, drill-seeding and funds are needed when working with these heavily disturbed lands.
Knowing the site history is critical to restoration success, and using more tools than simply weed-suppressive bacteria is likely needed for restoring abandoned farmland. ![]()
PHOTO 1: This rangeland site was burned in the spring of 2015, bacteria was sprayed in December 2015, and the picture was taken September 2016. The bacteria was sprayed along the fence row, from the first fencepost and to the right of the fence. Cheatgrass growth in the control fills the area, while there are few cheatgrass plants growing in the area sprayed with bacteria. The bacteria reduced the number of cheatgrass plants by 96 percent.
PHOTO 2: Pseudomonas fluorescens applied to agar in Petri dish.
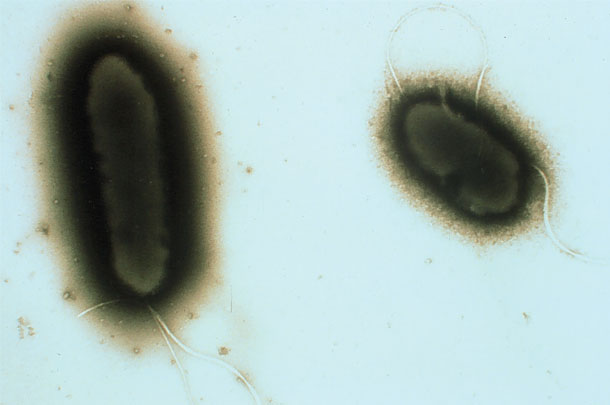
PHOTO 3: Transmission electron micrograph of Pseudomonas fluorescens.
PHOTO 4: Cheatgrass seed after five days on agar Petri dish. The untreated control (left) seeds have germinated and are producing roots and shoots. Seeds treated with weed-suppressive bacteria (mid, right) have no or stunted roots and shoots.
PHOTO 5: Rangeland field plots at Park Valley, Utah, of untreated control (left) and plots treated with the bacterium Pseudomonas fluorescens strain ACK55 (right) three years after application of the bacteria. The bacteria reduce cheatgrass populations by 56 percent. Photos provided by Ann Kennedy.
Want to learn more? Email Anne Kennedy or calling her at (509) 335-1554.

-
Melissa Bravo
- Certified Crop Adviser and Herd Health Specialist
- Meadow Lake Farm Consulting
- Email Melissa Bravo









