Recently, on an online forum, someone asked how much nitrogen everyone was applying on their triticale fields to get an idea of what application rate to use.
Ideally, representative soil samples should be submitted to a laboratory for analysis to accurately determine the need for nitrogen and all the macro- and micronutrients. The physical, biological and chemical characteristics of a particular soil (field) are unique; the same can be said about silage.
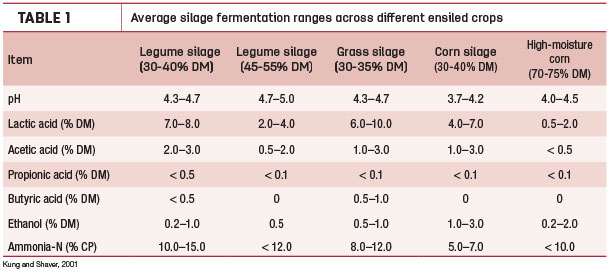
Ranges of typical values for fermentation products in silages can be found in the literature (Table 1), although it is common to get results that deviate.
An extensive range of factors impact the ensiling process, from the specific microenvironment where the crop is grown to the ability of keeping air out of the forage mass during the entire ensiling process. This article will discuss a recent silage case study that negatively affected animal performance and how laboratory analyses were used to help troubleshoot the problem.
The situation
A consultant for a 2,000-milking-cow dairy overseas was concerned about reduced animal intake and milk production. The triticale silage was among the factors potentially responsible for the issue, requiring an in-depth investigation. The crop came from many different fields, including some irrigated with effluent.
When observed on-site, the silage seemed to have undergone a successful fermentation, with no visual signs or smells of a poor or clostridial fermentation.
However, animal intakes started to drop as the silage was fed; the feedout rate was further reduced because of tight feed inventories which led to aerobic stability issues and feed spoilage. Spoiled silage was discarded, but intakes and production did not recover.
Interpreting laboratory analyses
The initial fermentation analysis showed 32 percent dry matter (DM), pH 5.51, total silage acids 10.7 percent DM, soluble sugars 5.7 percent DM, ammonia-N 7.3 percent, crude protein and ash 8.7 percent DM. Let’s go through these key fermentation parameters in more detail.
The pH is a measurement of the silage acidity, and it is correlated to the amount and proportions of the organic acids produced.
The initial analysis reported a pH higher than desired. Some reasons for an unusually high pH are high overall silage DM level, unfermented silage (short or no storage time, cold temperatures, etc.), very high ash and protein levels, especially in legume silages, excess ammonia-N or clostridial/spoiled/moldy silages. However, this particular sample presented high pH and no evidence of other potential factors.
While the total concentration of acids appeared sufficient, this did not tie in with the high pH, and the level of residual sugars indicated no substrate limitation to the fermentation. Individual fermentation acid levels were not available on the first analysis. The ash concentration did not appear to be a cause for concern.
Typically grasses range from 6 to 8 percent DM ash; clearly excess ash from soil contamination (weather, plant lodging, mechanical operations during harvest, soil from dirty pads) was not a component in this situation.
Looking at the protein fractions, the concentration of ammonia-N was below the red flag value of 10 percent crude protein, although the amount of soluble protein was high (86.8 percent DM). Excessive amounts of ammonia-N are a consequence of high protein breakdown and are linked to a slow pH drop, low-DM forages and silage filled too slowly or packed too loosely.
Ten days later, an analysis report with the individual fermentation acids became available, and it showed quite a messy profile: lactic acid 3.1 percent DM, acetic acid 6.21 percent DM and butyric acid 1.42 percent DM. Still, the high pH did not fully tie in with the amounts of lactic and acetic acids present.
Additionally, there was a considerable amount of butyric acid, but there was not a high level of ammonia-N, which is usually concomitant with the presence of butyric acid, and local representatives again confirmed there was no classical foul odor due to clostridial (butyric) fermentation.
The most desirable fermentation during the initial ensiling period is homolactic, in which only lactic acid is produced by the fermentation of the main plant sugars. Lactic acid is a strong acid that leads to a fast pH drop with the lowest DM and nutrient loss possible. The production of moderate amounts of acetic acid when the forage is inoculated with Lactobacillus buchneri are also important because acetic acid has stronger antifungal properties than lactic acid and is known to control spoilage micro-organisms and enhance feedout stability.
High levels of butyric and acetic acids in silages are normally the result from a slow fermentation leading to a clostridial fermentation. Butyric silages should not be fed to transition, early lactation cows and should be limited to no more than 50 grams of butyric acid per animal per day for replacement heifers, far-off dry cows and late-lactation cows.
As mentioned, the low level of ammonia-N did not support the hypothesis of clostridial activity, so other undesirable micro-organisms could have been involved.
To further investigate the situation, another round of samples were obtained at different depths from the silo face and sent out to a U.S. forage laboratory for more in-depth analyses.
The new results showed no difference in DM levels, protein fractions, ash and soluble sugar contents. Other parameters of note reported were pH 4.0, lactic acid 5 percent DM, acetic acid 3.5 percent DM, zero percent butyric acid and the presence of 2,3-butanediol and succinic acid. Additionally, the feed was tested for biogenic amines, and as much as 700 parts per million were found, mainly from cadaverine, putrescine and tyramine.
The absence of butyric acid contradicted the initial fermentation acid analysis, although it did tie in with the lack of butyric smell in the silage and the relatively low ammonia-N. The presence of 2,3-butanediol and succinic acid is indicative of enterobacteria activity, which could also be involved in the production of the biogenic amines by decarboxylation reactions.
Biogenic amines are not typically found in silages and have been negatively associated with intake by reducing ruminal motility and silage palatability.
By the time the in-depth analyses were available, it was about a month after receiving the first laboratory results, and the animal intakes had recovered. The farm is well-known for spraying effluent on crops, even when very close to silage harvest, and the challenges of this practice were explained in the drop in animal intake and performance, as well as the parameters revealed by the more in-depth analyses.
Key takeaways from this experience
- Always keep accurate and detailed records of your silage crop history.
- Putting up forage from different fields leads to inconsistencies in the silage.
- Carefully manage field fertilization with manure/effluent; ensure applications are rained or watered.
- A very stable silage does not mean it is static – changes do occur even during storage.
- Send in samples to a credible laboratory for analysis.
- If something does not make sense (even a feed analysis result), investigate it further.
PHOTO: Testing the silage. Courtesy photo.
Bob Charley has a Ph.D. in applied microbiology from the University of Strathclyde in Glasgow, Scotland. Renato Schmidt has a doctorate in animal nutrition from the University of Delaware and is employed by Lallemand Animal Nutrition as a forage products specialist.
References omitted but are available upon request. Click here to email an editor.

-
Bob Charley
- Forage Products Manager
- Lallemand Animal Nutrition, North America
- Email Bob Charley