“The thoughtless person playing with penicillin treatment is morally responsible for the death of the man who succumbs to infection with the penicillin-resistant organism.
I hope this evil can be averted.”
—Sir Alexander Fleming, Scottish physician and microbiologist who discovered penicillin
These are powerful words from the man whose discovery essentially revolutionized modern medicine. In developed nations, infectious disease went from the leading cause of death to a treatable condition.
Surgeons were able to develop ever-more-complicated surgical operations as antibiotics prevented life-threatening post-operative infections. Finally, mankind had won the battle against pestilence and disease … or so we thought.
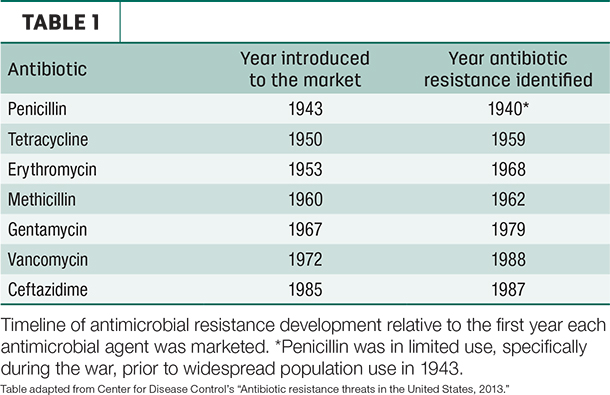
Just as Sir Alexander Fleming predicted, our “wonder drugs” started being less effective. Table 1 outlines the relatively short time it has typically taken to identify antimicrobial resistance (AMR) following widespread introduction of antibiotics.
What is AMR?
When we talk about AMR, we’re referring to the phenomenon by which micro-organisms (bacteria, fungi, parasites and viruses) develop the ability to survive in the presence of the compounds designed to kill them or limit their growth.
These micro-organisms (for our discussion, we’ll focus on bacteria) survive and are able to pass on their resistance machinery to other bacteria, potentially spreading resistance across different populations and species of bacteria (and potentially between animal and human).
Why is AMR important?
It’s estimated over 2 million illnesses have been caused by AMR infections in the U.S. alone, resulting in approximately 23,000 deaths. When turning focus to world numbers, that number balloons to 700,000 deaths due to AMR infections.
Indeed, if AMR continues unchecked, by 2050 approximately 10 million people could die from an AMR infection. Essentially, if action is not taken immediately to address the issue, we could go back in time to a pre-antibiotic era.
Why worry in dairy?
AMR is not just a problem in hospitals. Animal agriculture uses vast quantities of antimicrobial agents every year to treat and prevent disease; some 80 percent of all antibiotics produced are used in livestock.
Granted, a high proportion of this figure is monensin (a growth-promoting drug not used in human medicine), but there is still a significant proportion of antimicrobials used of critical importance in human medicine.
AMR bacteria can be transmitted between animals and humans in several ways (Figure 1).

We know people in direct contact with treated animals are at a higher risk of transmission. In addition, AMR bacteria could make their way (at low levels) to the animal products we consume and the environment at large, thereby increasing the reservoir of resistance and the potential for transmission.
While there is a potential link between the development and transmission of AMR in animals to humans, there is no data characterizing the magnitude of the risk relative to other potential sources. Regardless, everyone who uses and prescribes antibiotics shares a role in the development and implementation of solutions.
Drug-resistant Salmonella Dublin: A threat to humans and cattle
In recent years, multi-drug-resistant Salmonella enterica serotype Dublin has emerged as a major cause of illness in dairy youngstock. Signs in calves range from high rates of fever, respiratory disease, poor response to antibiotics and mortality.
Cattle are the primary reservoirs for these bacteria – often carrying and shedding the bugs without showing clinical signs. One big concern is zoonosis (infection from animals to humans) because cattle and their meat and milk products are the primary source for S. Dublin infection in humans.
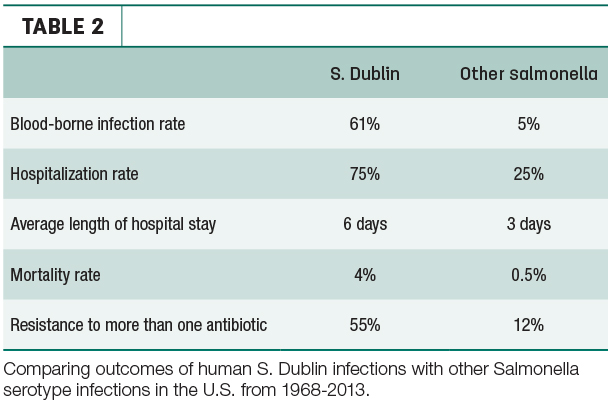
Though the incidence rate of S. Dublin is low (roughly 0.042 cases per 100,000 persons), the rate was nearly eight times higher in 2013 than it was in 1968, all while the incidence of other serotypes of salmonella have remained stable.
What’s worse, relative to other serotypes of salmonella, S. Dublin infections are more harmful to people (Table 2). The risk is especially high for those who consume raw milk – one more reason to pasteurize.
 Focusing on biosecurity practices to limit S. Dublin introduction and spread, as well as judicious use of antibiotics across the farm, are necessary to contain the development and spread of multi-drug-resistant S. Dublin.
Focusing on biosecurity practices to limit S. Dublin introduction and spread, as well as judicious use of antibiotics across the farm, are necessary to contain the development and spread of multi-drug-resistant S. Dublin.
Every time we go to the medicine cabinet and treat a cow with antibiotics, we’re potentially selecting for resistant bacteria that can survive and pass on their resistance. Every dose has a consequence.
What can we do about it?
1. Paradigm shift. First and foremost, we need to rid ourselves of the thought this is someone else’s fault. Everyone who uses antibiotics needs to do so in a responsible manner to ensure drug effectiveness for years to come.
2. Reduction in use. The most ambitious, far-reaching and thorough study to date was published recently, and it evaluated the available literature to assess the effects a reduction in antimicrobial use in animals has on AMR in both animals and humans.
Specifically, restricted antibiotic use resulted in a 10 to 15 percent reduction in prevalence of AMR in animals and a 24 percent reduction in AMR in humans (primarily humans in contact with farm animals). Reducing use does make a difference.
3. Avoid use of critically important antimicrobials. These are the drugs used to treat the most aggressive infections in humans. If they stop working, there is nothing else to use. These drugs include third-generation cephalosporins, fluoroquinolones, macrolides and polymyxins.
The World Health Organization recommends these drugs not be used in food-producing animals so as to reserve them for use in humans. In dairy, the only drug used extensively is ceftiofur; this drug has zero milk withhold and is used to treat a variety of infections including pneumonia, metritis and foot rot.
Luckily, there are many alternatives, such as penicillin, amoxicillin and tetracycline. Appropriate therapy for a given situation should be discussed with your veterinarian.
4. A strong consultative relationship between veterinarians and producers.
- Preventing infections is key in reducing the need to use antibiotics. Strategies that keep things clean, comfortable, calm and consistent go a long way in ensuring health.
- Standard operating procedures should be systematic, thorough and evidence-based with an eye for evaluating and maintaining compliance.
- Increased use of diagnostic testing to ensure antibiotics are warranted, and, if so, what choice should be made.
- Treating healthy animals with antibiotics has become an unacceptable practice, and blanket dry cow therapy is the big area in dairy production that could be improved. When used with an internal teat sealant, selectively treating only those cows infected at dry-off is an effective means of reducing use without compromising udder health.
“Antibiotic resistance is a habit. We all know how hard it is to change a habit. But, as a society, we’ve done that in the past. People used to … not wear seat belts, smoke inside public buildings. We don’t do those things anymore … we decided those things were expensive, destructive, not in our best interest. We changed social norms. We could change social norms around antibiotic use, too.”
—Maryn McKenna, public health journalist, TED talk, March 2015
We are living in an interconnected and interrelated world. From physicians and patients to producers and veterinarians, on to politicians, bureaucrats and the public at large, we all use antibiotics; therefore we all have a role to play in solving the critically important problem of AMR. ![]()
Dan Shock is a associate consultant with Acer Consulting. Email Dan Shock.






