The U.S. Food and Drug Administration over a series of years has implemented new rules that will change how beef producers use antibiotic drugs for the care of livestock.
With the introduction of two new rules – called Guidance for Industry documents 209 and 213 – the agency created a line of distinction between antibiotic use for growth promotion and the less controversial uses of disease prevention/control and therapy.
Mike Apley, DVM and professor of clinical sciences at Kansas State University, says that with those three main categories, the FDA began targeting the use of medically important antibiotics used for growth promotion.
“Don’t think for a minute that it’s regulatory or legislative pressure that’s the biggest changer. The biggest changer is going to be retail supply chains reacting to what they think consumer desires are.”
H. Morgan Scott, professor and veterinary epidemiologist at Texas A&M, agrees with the changes coming from the demand side. But he adds that producers are also following a sense of morality and ethics with antibiotic reform.
“Science comes down to this: Less is better, but zero is not an option,” Scott says. “We know that the less of an antibiotic we use, the slower resistance will rise.
“But you can’t not treat animals. We have a moral obligation to treat sick animals we’re in care of. So the judicious use comes into using this limited resource as best as possible in the future.”
Restricting antibiotics for growth
The first principle created with Guidance for Industry 209 is for medically important antimicrobials in food-producing animals to be limited to purposes of animal health.
Using antibiotics for growth will be “voluntarily withdrawn from the instruction labels of drugs by the companies,” Apley says.
But the FDA’s delineation between growth promotion uses and prevention/control uses is problematic, Apley says, as some treatment regimens for both control and growth have some overlap.
But given the rising opposition to growth promotion antibiotics, caution will rest on eliminating their use, Apley says.
Looking ahead, the FDA could begin targeting routine prevention/control uses of antibiotics, and even further in the future, it could also limit therapy uses, Apley says.
Apley says even more troubling is the latitude the FDA gave itself in both documents by stating “it is not limited to making risk determinations based solely on documented scientific information but may use other suitable information as appropriate.”
“What that tells us is our future of antibiotics in food animals is not relegated to scientific information. That’s very, very clear.”
Requiring veterinary oversight
The second principle created with GFI 209 is that using medically important antimicrobial drugs in food animals “should be limited to those uses that include veterinary oversight or consultation.”
Apley says this principle will require significant change in production practices and more training and involvement with a local vet.
“The days of being able to walk in and buy medicated milk replacer, a bag of medicated feed for show animals or a bag of (aureomycin) crumbles are gone in December 2016,” Apley says.
“You will have to have a veterinary authorization for that in the form of a prescription if it goes in water, or a veterinary feed directive (VFD) if it’s in the feed.”
Producers will have to change their standard practices of buying routine medicines and antibiotics, including penicillin, to include veterinary prescriptions. The reforms taking place by December 2016 will also require VFD and prescriptions to avoid extra-label use of drugs.
Using penicillin as an example, Apley says if an operator uses it every day, that will require a prescription and must be monitored for the right dosage.
“If residue happens and they come and find that you were using it at 5 ccs per 100 outside of a vet-client patient relationship, and without a veterinary authorization, that is extra-label use by a layperson outside of the VCPR and would be an illegal use.
“When that will come bite you is with any residue issue ... That’s why the veterinarian in that relationship is responsible for assigning you an exaggerated withdrawal time.”
Determining ‘medically important’
Producers may ask just who is determining which antibiotics given to livestock also have the label of “medically important” to human health care.
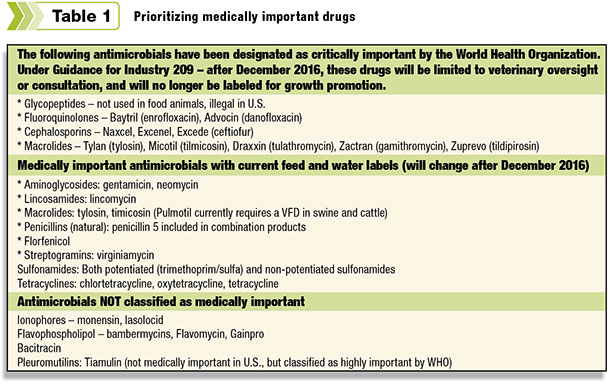
The Guidance for Industry 152 contains a list determined by an expert FDA panel called the Center for Drug Evaluation and Research (CDER). The panel focuses on human approval of drugs and also uses lists developed by the World Health Organization (WHO).
Click here or on the image avove to view it at full size in a new window. (PDF 2.7MB)
The WHO list places its highest priority on glycopeptides, fluoroquinolones, cephalosporins and macrolides – with macrolides having the highest medical importance. (See Table 1).
Meanwhile, the use of ionophores, Flavophospholipol and Bacitracin are not deemed medically important, and their use in feed will not change.
Apley says some antimicrobials already cross over between regimens of growth and control.
Scott says a drug is deemed medically important based on when it was discovered.
“The drugs discovered in the ’50s, ’60s, ’70s and early ’80s don’t tend to be critically important because resistance arose to them a long time ago. It’s the drugs more recently introduced that become critically important – because that’s all we have left.”
When a new class of drug comes on the market, it should be considered critically important, Scott explains. Progressively after drugs are introduced, resistance doesn’t tend to emerge for another decade.
The problem, however, is that new antimicrobials have not been developed, making the existing antimicrobials more medically critical than ever.
“There hasn’t been a new class since the ’90s, or a new class in veterinarian agriculture since 1978. That’s almost 40 years.”
An industry change on labels
In order to make GFI 209 and its two main principles effective, the FDA issued another document to make sure drug labels reflect the changes in use for antibiotics.
GFI 213 came out in the summer of 2013, Apley says, and “gave a roadmap for companies to fulfill the voluntary term of Guidance 209.”
GFI 213 recommends companies comply with Guidance 209 by removing label indications for growth promotion and inserting label requirements for veterinary authorization. In exchange for doing that, the companies will not have to redo any other label claims for microbial safety, Apley says.
Critics hammered this document, saying because it was a voluntary rule, it would be toothless in changing how antimicrobials are used.
But that has been disproved, Apley says. Three months after its release, GFI 213 got the approval of all 26 drug companies for all 283 labels. Again – the December 2016 timeline has significance. That’s the date all companies will change all labels to cooperate with FDA rules.
Preparing for change
Apley says the veterinary feed directive and rules requiring more veterinary oversight require a longer perspective of planning for operators, and communication with a licensed vet they trust will be critical.
Electronic systems are being developed “that will allow a vet to keep all your information stored.” Vets will update and renew information for clients and send it to the retail point it is sold – just like a standard medical prescription.
Apley says states will be responsible for defining veterinarian oversight and creating the standard details for prescriptions.
Vets still need to specify duration of use, number of animals to be treated and the level of the VFD drug in the feed. The vet community, Apley says, wants a standard VFD duration to be six months.
But producers in the future need to prepare themselves for more rigid standards as well.
“Then they’ll go into it at the required time and renew it … and hopefully can send electronic copies to who you are buying from.
“Be prepared for discussions,” Apley says. “Veterinarians now aren’t just going to be responsible for the antibiotic use; they are accountable. We are working hard as a veterinary profession to bone up on justification for all the uses, what the evidence is for efficacy, when they are going to do you good.
But don’t expect it to be: ‘I want this, I want you to write it.’ I hope everyone approaches that as a conversation.
“If it gets vets and producers talking more about what’s actually going on in a place, that’s a good thing.” ![]()
Watch an interview with Mike Apley.
PHOTO
Mike Apley of Kansas State University. Photo by David Cooper.
Antibacterial vs. antibiotic
- Antibiotics: A natural compound created by one living micro-organism to inhibit or kill the growth of another.
- Antimicrobials: Includes all classes of antibiotics as well as synthetic or chemically created compounds.