The short answer: All feed-use antibiotics the FDA, World Health Organization and Center for Disease Control (CDC) consider “medically important to humans.” Currently, the FDA has approved one VFD antibiotic, tilmicosin (Pulmotil), for use in cattle feed to control bovine respiratory disease (BRD).
Medically important antibiotics currently being used in cattle feeds that have label indications for prevention, treatment or control of specific bacterial disease as required by the VFD regulations, but will require new approvals by the FDA to continue the feed antibiotic use when the VFD regulation becomes effective in December 2016, include:
- Chlortetracycline (Aureomycin, CLTC, Pennchlor)
- Chlortetracycline + Sulfamethazine (Aureo S 700)
- Neomycin + Oxytetracycline (Neo-Terramycin, Neo-Oxy)
- Oxytetracycline (Terramycin, Pennox)
- Tylosin (Tylan)
- Virginiamycin (V-Max)
What products don’t require a VFD?
VFD regulations focus on “medically important antibiotics,” as these represent the only medication type identified that the use in livestock feed could potentially jeopardize the drug’s effectiveness in humans. Therefore, medications used to control parasites, reproduction, bloat, etc., will not require a VFD. These include:
- Amprolium (Corid)
- Bacitracin (Albac, BMD)
- Bambermycin (Gainpro)
- Decoquinate (Deccox)
- Fenbendazole (Safe-Guard)
- Laidlomycin (Cattlyst)
- Lasalocid (Bovatec)
- Melengestrol acetate (MGA)
- Methoprene (Altosid)
- Monensin (Rumensin)
- Morantel (Rumatel)
- Poloxalene (Bloat Guard)
- Ractopamine (Optaflexx, Actogain)
- Tetraclovinphos (Rabon)
Can a VFD medication be used in a feed that contains other FDA-approved feed additives?
Yes, provided the FDA has approved the medications to be used together in the same feed. For decades, the FDA has approved combination use of monensin (Rumensin), tylosin (Tylan) and melegestrol (MGA) in the same feed.
Tilmicosin (Pulmotil), the only VFD antibiotic currently approved by the FDA, is approved for combination use with monensin. There is no reason to think the FDA will not continue to approve combination use of VFD medications approved in the future with other FDA-approved feed medications.
Examples of the use of the currently approved VFD we will refer to as ‘T-antibiotic’
Example: newly weaned calves
- Cattle producer contacts their vet about newly weaned calves developing pneumonia and indicates that at least 10 percent are showing the DART signs (depression, loss of appetite, respiratory movement changes and an elevated temperature).
These were signs they and their vet had previously discussed as keys to early diagnosis of pneumonia.
- Their vet writes a VFD using the form provided for T-antibiotic.
- The forms include the name, address, phone of the cattle owner and the veterinarian.
- The location of the cattle is noted on the form.
- The form also indicates how many cattle are to be covered by the VFD and their approximate weights.
- The amount of VFD medication that will be needed is calculated and recorded on the form (not required in the final rule).
- A note is included if the VFD drug is to be used in combination with another drug as approved by the FDA.
- The “effective date” of the VFD and the “expiration date” as calculated from the effective (start) date is listed.
- The required withdrawal time is recorded on the VFD form.
- The veterinarian signs the form and provides a copy for both the cattle producer and for the feed distributor that will provide the VFD medication.
- For this example, the VFD for T-antibiotic has a 45-day expiration date calculated from the VFD effective date listed on the VFD form by the veterinarian.
This means the VFD therapy must be initiated within the 45-day window from the VFD effective date to the VFD expiration date … but that allows for a complete therapeutic cycle to be completed.
- The VFD-approved T-antibiotic will be fed to 100 calves that average 500 pounds.
- The VFD for T-antibiotic requires that at least 10 percent of the cattle in the group are exhibiting signs of pneumonia.
- The VFD T-antibiotic is fed for 14 continuous days, and a 28-day withdrawal is assigned at the end of the 14-day treatment.
- To meet the 45-day VFD expiration window, the T-antibiotic must be started not later than 31 days from the VFD effective date (45-day window - 14 therapy cycle = 31-day window to start a therapy cycle).
- Three concentrations of T-antibiotic are available for on-farm mixing. For this example, a “Type B article” 5.68-gram-per-pound product will be used. This is especially convenient for this example as the dose is 5.68 mg per pound of calf.
Therefore, each pound of 5.68-gram-per-pound Type B product will medicate two 500-pound calves each day during the 14-day treatment. The total amount of Type B product to be used for the 100 calves weighing 500 pounds for the entire 14-day treatment will be 700 pounds.
- The ration being fed to the calves is 75 percent dry matter.
- T-antibiotic has a few use restrictions that must be followed.
- Cattle in the group that are exhibiting clinical signs of pneumonia should be removed for individual treatment.
- An injectable antibiotic in the same macrolide class cannot be used in conjunction with or prior to the use of the T-antibiotic.
- The T-antibiotic must be used in a complete feed, which means no other feed or feedstuff can be offered.
- Cattle must receive between 1.5 and 2 percent of their bodyweight of the complete feed on a dry matter basis.
- No other feed can be offered while feeding the VFD-medicated feed (no extra hay or lick tubes).
- The feed mixer used to prepare the complete feed should be “flushed out” before being used to mix feed not containing the tilmicosin.
- Between 1,000 as-fed pounds to 1,333 as-fed pounds of the 75 percent dry matter complete ration will meet the 1.5 to 2 percent daily intake restriction for the 100 5-hundredweight calves required by the VFD for T-antibiotic.
- The amount of the T-antibiotic 5.68 gram per pound of the Type B product used daily for the 100 5-hundredweight calves will be 50 pounds. This amount will be added to the 1,000 to 1,333 pounds of as-fed daily feed delivery.
Example: feeder planning on purchasing several loads of high-risk calves in the next month
- A vet’s client, John Doe, is planning to buy 10 100-head loads of 5-hundredweight high-risk calves starting the first of next month (example, Aug. 1), with the last load coming in during the next four weeks.
- Vet needs to contact the VFD medication supplier (example, feed store) to let them know John Doe will be using a VFD medication and what concentration of the medication they want to use. It may take the supplier one to two weeks to get the medication in stock.
- John Doe’s vet (licensed in the state where the cattle will reside and as a VCPR) can write a VFD for T-antibiotic and, as a “special instruction,” indicate the VFD to begin Aug. 1, 2016.
- The expiration date for T-antibiotic is labeled as 45 days. In this example, the VFD usage window will be from Aug. 1 to Sept. 14, which means all feeding of T-antibiotic on this VFD must stop at midnight Sept. 14.
- T-antibiotic has a 14-day feeding cycle. Therefore, all groups for cattle identified to receive T-antibiotic must be started on a treatment cycle by Aug. 31.
- If there is any delay in receiving the anticipated 10 loads of cattle, a second VFD will need to be written to extend to potential treatment past the expiration of the original VFD (Sept. 14).
- The dose of T-antibiotic is 5.68 mg per pound per day. T-antibiotic is available in a 5.68-gram-per-pound pellet. Therefore, the dose would be 1 pound of these pellets per 1,000 pounds of cattle being treated.
- T-antibiotic has couple of important feeding restrictions.
- Must be fed to cattle consuming feed between 1.5 and 2 percent of the bodyweight on a dry matter basis
- No other feedstuff can be offered … not extra hay, lick tubes, etc.
- The total amount of T-antibiotic that will be needed for a group of 100 5-hundredweight calves will be:
- 1 pound of pellets will treat 1,000 pounds of cattle per day
- 5 hundredweight x 100 head = 50,000 pounds … 50,000 pounds / 1,000 pounds = 50 pounds of pellets per day
- 50 pounds of pellets x 14 days therapy cycle = 700 pounds of T-antibiotic pellet (5.68 grams/pound)
- The total amount of feed that is 65 percent dry matter fed to a group of 100 cattle weighing 5 hundredweight would be:
- Feed intake can range between 1.5 to 2 percent of bodyweight on a dry matter basis.
At 1.5 percent bodyweight (dry matter basis) = 500 x 1.5 percent = 7.5 pounds (dry matter basis) daily feed
7.5 pounds / 65 percent = 11.5 pounds as-fed feed per day
11.5 pounds daily x 14 days = 161 pounds as-fed per head x 100 head = 16,100 pounds as-fed feed
At 2 percent bodyweight (dry matter basis) = 500 x 2 percent = 10 pounds (dry matter basis) daily feed
10 pounds / 65 percent = 15.4 pounds as-fed feed per day
15.4 pounds daily x 14 days = 216 pounds as-fed per head x 100 head = 21,600 pounds as-fed feed
- Both the total amount of VFD medication to be used and the total amount of feed it will be mixed with must be recorded on the VFD form.
- All parties (vet, VFD medication supplier and producer) must have a copy of the completed VFD form. These copies must be retained for two years.
- The VFD medication supplier (feed mill) must have a copy before the medication can leave their premise. It can be a fax or email copy, but a hard copy must be provided to the VFD medication supplier within five working days.
- Note: If the VFD supplier is new to working with VFD medications, they must notify the FDA of their intent to distribute VFD medications. This is a “one-time” notification.
Once the notification has been submitted, the supplier will not have to repeat the process, even if a new VFD product becomes available to distribute.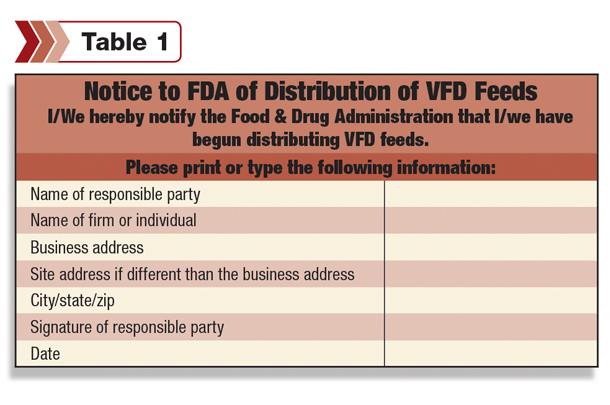
- Example of a form for notifying the FDA (Table 1).

-
Dee Griffin
- Veterinarian, Professor
- Great Plains Veterinary Education Center
- Email Dee Griffin







