“Drug misuse on dairy cows prompts federal warning,” Star Tribune, Nov. 21, 2009 Newspaper headlines such as the above are becoming too common. The Star Tribune published an article in November 2009 recounting the reprimand of two local Minnesota dairy producers by the Food and Drug Administration (FDA) for sending cows to slaughter with “dangerously high” violative drug residue levels in their system.
In March 2010, a U.S. District judge issued an order barring a New York dairy farmer from selling his cattle to be slaughtered for human consumption until he complies with federal limits on antibiotic residues. The USDA said the farmer violated the law six times in the past 10 years by failing to keep adequate records of which cows were medicated. While some may consider these news reports biased and based on misinformation, we do need to step back and look at our current practices and work together to solve this problem.
Why is the issue of antibiotic residues in cull dairy cattle important?
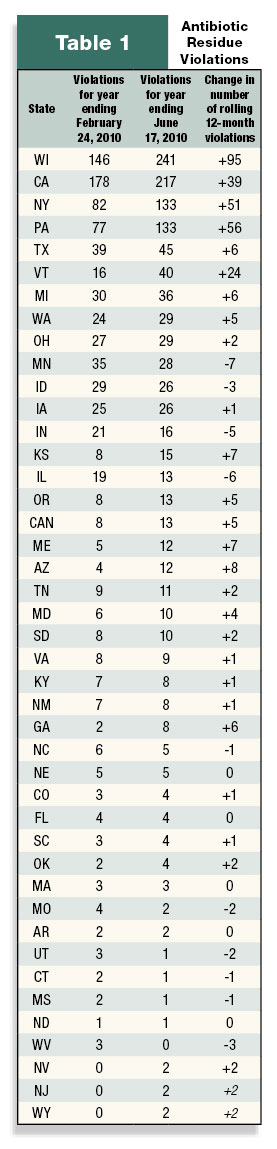
In 2008 dairy cull cows accounted for 8 percent of all cattle harvested (excluding veal) at federally inspected plants. While cull dairy cows are a small part of total beef production, they carry a heavy burden and are responsible for more than 90 percent of cattle residue violations (excluding veal) from inspector-generated samples. In 2008:
• 33,805,100 cattle (excluding veal) were slaughtered in federally inspected plants
• 879 suspect cattle tested positive for a violative antimicrobial residue (0.003 percent of cattle slaughtered)
• 791 of the positive cattle were cull dairy cows (90 percent of suspect cattle that tested positive for an antimicrobial violation at slaughter)
Violative antimicrobial residues were detected in 0.03 percent of all dairy cows slaughtered. In comparison, violative residues were detected in 0.0001 percent of all beef cows slaughtered. So, while the percentage of violative residues detected in harvested dairy cows appears to be small, it is 300 times greater than the percentage of violative residues detected in harvested beef cows (numbers adapted from the USDA’s 2008 National Residue Program data).
Issues that affect the quality and safety of beef that originates from cull dairy cattle are an ongoing concern for the beef industry. Any level of violative residue is unacceptable in the food industry.
Legislation is being considered in Washington, D.C, which would withdraw the routine use of seven classes of antibiotics from food animal production unless animals or herds are sick or unless drug companies can prove that their use does not harm human health. The effort has support of the American Medical Association, the American Public Health Association, the Infectious Diseases Society of America, the American Academy of Pediatrics and some 350 other organizations.
Legislative attempts to severely restrict or eliminate the use of antimicrobials in livestock are not the answer to these issues. Antimicrobials and other pharmaceuticals are an important tool in the production of safe and wholesome food such as beef. Restricting the use of these important tools has the potential for negative effects on animal health and welfare as well as human health. We in the dairy industry must recognize that we have a dual role as both dairy and beef producers, and a responsibility to the public, for the safety of the beef that enters the food chain.
Why are dairy cattle treated with antibiotics?
Antibiotics are an important and valuable tool in any good herd health program. Antibiotics, used according to best practices, protect the health of the herd and, by extension, human health. One of the primary uses of antibiotics in the dairy industry is for dry cow treatment. Nationwide data indicate that approximately 90 percent of operations in 2007 used intramammary antibiotics at dry-off, most commonly Penicillin G (procaine)/dihydrostreptomycin and cephapirin.
Antibiotics also have an important place with respect to the treatment of disease in individual animals. Mastitis, followed by respiratory disease and lameness, are the three most common diseases for which antibiotic treatments are used on dairy operations. Nationwide data for 2007 indicate that cephalosporin was the most common type of antibiotic used by operations to treat all diseases, while B-lactam antibiotics, such as penicillin, were the second-most common type of antibiotic used to treat mastitis and reproductive diseases, and the third- most common type of antibiotic used to treat lameness. This usage mirrors antibiotic residue data reported by federal inspectors using on-site antibiotic residue testing, in which penicillin was identified as the most common violative residue detected in cull dairy cows.
What are the potential causes of antibiotic residues?
Antibiotic residues are, in most cases, unintentional and result from on-farm circumstances that can be easily addressed. Common situations leading to unintentional misuse of antibiotics may include insufficient knowledge about drug withdrawal periods, employee error, insufficient treatment records, poor identification of treated animals and inadequate communication between veterinarian and producer.
A recent survey of antibiotic use and biosecurity practices among Washington State dairy producers highlights many of these underlying gaps. Out of 381 respondents, less than one-third had written protocols for diagnosing or treating common medical conditions. Most agreed that such protocols could reduce errors and production losses. In a University of Minnesota survey, more than 18 percent of bovine practitioners surveyed cited poor communication between veterinarian and producer as a major reason of dairy producers’ lack of compliance with drug use instructions that could potentially cause a violative residue. Lack of understanding of residue avoidance practices was cited as the second-most common reason for non-compliance by producers.
What is the solution?
Responsible use of antimicrobials and pharmaceuticals is the answer. A veterinary-client-patient relationship (VCPR) is paramount to ensuring that antibiotics are used according to best management practices. A VCPR is not only required by law for the use of prescription veterinary drugs, but is also a valuable resource for developing protocols for routine herd management and for clinical therapy and discussion about proper drug use, withdrawal time, antibiotic resistance and disease prevention practices.
As defined in the Principles of Veterinary Medical Ethics of the American Veterinary Medical Association, a VCPR exists when all of the following conditions have been met:
1. The veterinarian has assumed responsibility for making clinical judgments regarding the health of the animal(s) and the need for medical treatment, and the client has agreed to follow the veterinarian’s instructions.
2. The veterinarian has sufficient knowledge of the animal(s) to initiate at least a general or preliminary diagnosis of the medical condition of the animal(s). This means that the veterinarian has recently seen and is personally acquainted with the keeping and care of the animal(s) by virtue of an examination of the animal(s), or by medically appropriate and timely visits to the premises where the animal(s) are kept.
3. The veterinarian is readily available, or has arranged for emergency coverage, for follow-up evaluation in the event of adverse reactions or the failure of the treatment regimen.
What is being done to address this issue?
The University of Minnesota’s College of Veterinary Medicine has developed and piloted a new software package to aid in writing protocols and producing veterinary prescriptions under a valid VCPR. Version 1.0 of the new Veterinary Protocol Manager software is expected to be available in late 2010 through the Veterinary Population Medicine Department at the College of Veterinary Medicine.
The Minnesota Veterinary Medical Association – Farm Animal Pharmaceutical Committee (MVMA–FAPC) works to keep veterinarian members up to date on issues related to pharmaceutical use in food-producing animals. The FAPC monitors current federal and Minnesota drug regulations for food animal practitioners, communicates new information to members and works to assist members with their efforts to be compliant with the current legal standards and guidelines surrounding the use and distribution of drugs for use in food animal species.
The Center for Animal Health and Food Safety and Minnesota Beef Council work in partnership to assist dairy producers and veterinarians in ensuring that dairy beef meet beef quality assurance (BQA) standards. Recently the CAHFS and MBC have developed brochures and posters on responsible antibiotic use, available in both Spanish and English. Visit the Minnesota Beef Council for these and other BQA materials at www.mnbeef.org or the national Beef Checkoff-funded site at www.bqa.org PD
References omitted due to space but are available upon request by sending an email to editor@progressivedairy.com .
Drs. Koeman and Goldsmith are with the University of Minnesota’s Center for Animal Health and Food Safety. Ronald F. Eustice is executive director of the Minnesota Beef Council.

-
Jennifer Koeman
- Veterinary Public Health Resident
- Center for Animal Health and Food Safety
- Email Jennifer Koeman





