Research conducted by Cornell Cooperative Extension before and after the first year of VFD implementation found the most common issue regarding the new requirements of veterinary-client-patient relationships (VCPR) and the VFD was difficulty obtaining in-feed antibiotics in a timely fashion after they were ordered by a veterinarian.
This research was based on interviews with dairy farmers as well as veterinarians and feed mills that primarily serve dairy farms. It was clear from the responses most of the dairy farms had adjusted to the new regulations fairly well, with most citing the greatest inconvenience as “additional paperwork.” However, this potential burden was mostly overshadowed by the requirements of the National Dairy FARM Program, which was mandated around the same time for farms shipping their milk to cooperatives that participate in National Milk.
Farms that reported “negative financial impacts” were mid- to large-sized farms, due to increased costs of treatments in calves or potential death loss. The farms reported situations where in-feed antibiotics were recommended for groups of animals due to respiratory outbreaks above the standard threshold of 15 percent and, as such, appropriately recommended by their veterinarian with whom they have a VCPR.
However, their local feed mills were not carrying enough in stock to supply the farm’s needs. This was a natural response to the VFD for the feed mills – the sales of the antibiotic in-feed products had dropped considerably. The feed mills indicated they still keep some stock on hand but are keeping less of those products on hand or mixing/making less of them.
The ultimate impact to the farm was having to wait anywhere from one to three weeks for the recommended feed with antibiotics to be ready. In that time span, there was the potential for additional animals to become infected, ill animals to degrade in condition and limited alternative options including the usage of more expensive and labor-intensive treatment options on a greater number of animals. It is important to note, on these farms, the lack of availability did not stop the farms from treating animals with necessary medications to treat infections – “doing nothing” was never an option.
The exact financial impacts of a management change on dairy operations are challenging to establish. Here we provide an example of the increase in short-term treatment costs and discuss more generally the potential long-term implications of an extended outbreak of respiratory disease. The immediate financial impact would be the cost difference between utilizing the in-feed antibiotics on a group of animals when it is recommended by a veterinarian and treating animals individually once they are presenting as physically ill with other antibiotic options.
While there are many variables associated with those two decision paths, it is important to consider, on most dairy farms, labor is the second-highest single expense after feed. Any management option that requires a considerable amount of additional labor, such as treating more animals individually, could lead to significantly increased costs.
As a simple example, it is not unusual for farms to treat with in-feed antibiotics when 15 percent of a pen presents respiratory clinical symptoms. In Table 1, we show the costs of treating both individual animals presenting with clinical symptoms and providing in-feed antibiotics for a pen of 100 heifers.
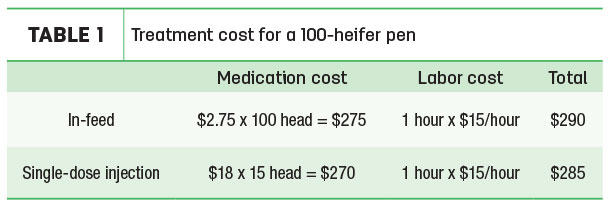
In this scenario, individual treatment with a single-dose antibiotic treatment can range from $14 to $23 per dose. While farm level costs vary widely, we present this as a typical example of the financial impacts of a disease typically treated with antibiotics.
Assumptions
- Pen of 100 400- to 500-pound, 4- to 6-month-old heifers, 15 percent or 15 heifers present with symptoms of respiratory clinical symptoms.
- In-feed antibiotics are available in a timely fashion, and no other animals present as ill.
- Each animal presenting with symptoms is treated with a single-dose antibiotic treatment. With a range of options available, we use $18 as a typical cost, although actual costs may be higher or lower.
- Labor costs of $15 per hour, includes benefits and skills necessary to administer treatment.
- For individual heifer treatment, time requirement is a half-hour of prep/dispensing/dosing before going out to treat animals and a half-hour of administering doses.
- In-feed antibiotic is a broad spectrum antibiotic for use in non-lactating dairy cattle. When fed as directed, these “crumbles” will treat bacterial enteritis caused by Escherichia coli and bacterial pneumonia caused by Pasteurella multocida organisms susceptible to chlortetracycline.
If the in-feed antibiotics could not be used in a timely fashion in the case of the respiratory outbreak presented in Table 1 (15 percent of pen initially presents as sick), there is a strong likelihood additional heifers could get sick over the subsequent one to three weeks.
Various factors are involved in further disease spread, including ventilation quality, stocking density, pathogenicity of the disease-causing organisms and immune competence of the at-risk population. In Table 2, we present the costs of individual heifer treatment if an additional 15 percent of the pen gets sick.
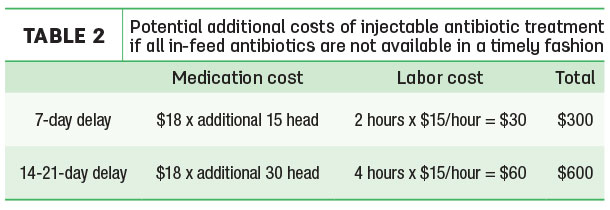
We also show costs in the case when 30 percent of additional heifers get sick over a longer period. Treating an additional 15 heifers costs $300, and an additional 30 would cost $600. These additional costs could be considered relatively modest – when over 15 percent of a pen presents with clinical respiratory symptoms, without timely in-feed treatment, another likely outcome is the entire pen becoming sick.
Assumptions
- Use single-dose antibiotic under same guidelines as in Table 1.
- Seven-day delay leads to an additional 15 percent of pen falling ill. Add additional two hours of labor costs for treatment of new cases. At this point, a total of 30 heifers have fallen ill, and the farm has spent a total of $585 for single-dose antibiotic treatments.
- Two- to three-week delay leads to another 30 percent of pen falling ill. Add additional four hours of labor costs for treatment of new cases. At this point, a total of 60 heifers have fallen ill, and the farm has spent a total of $1,185 for single-dose antibiotic treatments.
In the case 15 additional heifers become sick, the cost of additional individual treatments is roughly equivalent to initial treatment with in-feed antibiotics. This demonstrates if in-feed antibiotics are available and prevent illness, they are as cost-efficient as treating individual animals if 15 additional animals became ill (after the initial 15 become ill). Given this is a fairly likely outcome, and it is also common for an entire pen to get sick in this situation, this simple example demonstrates in-feed antibiotics can have short-term financial benefits in the case of high levels of respiratory outbreaks.
For this short-term perspective, if a high level of additional outbreaks are expected – for example, when isolation of sick heifers is not feasible, unavailability of in-feed antibiotics could cause an immediate but moderate financial loss for farms. Higher death loss in heifer pens, although likely limited to cases of severe respiratory outbreaks, is where the largest financial loss from disease is often incurred. However, given the cost of raising heifers is currently relatively close to the cost of purchasing them, it is important to evaluate all options for optimum heifer health.
Even if only a few additional animals became ill, there are unknown costs of lost future performance. Impacts on future milk performance may be much more significant, though it is difficult to quantify value. It is well established that illness, particularly respiratory stress during the growth and development stages, in dairy animals can significantly limit future milk performance. Every animal that escalates to clinically ill is potential negative financial impact to the farm.
One of the reasons this is difficult to measure is: Individual heifers that experienced illness may exit the farm before lactation. This in turn could mean the real “breakeven” point between using in-feed or individual antibiotic treatments could be much lower if future performance and earnings potential are considered. Indeed, one of the benefits of disease control strategies is the ability to manage subclinical disease.
Providing therapy to those animals exposed, potentially already infected but not showing obvious signs of disease, helps improve herd health, reduce animal suffering and minimize expense to the farm owner.
While this is one feasible outcome, there are a large number of additional variables that could affect these outcomes. The VFD does help with this because the veterinarian on-farm is more involved in herd health management decisions and can help assess those varying factors to determine the best course of treatment.
In our interviews on VFD changes, most farms indicated they are ultimately focusing on preventative care measures and other management techniques that would not require the usage of in-feed antibiotics, with the ultimate goal of reducing illness overall. Given that feed mills and veterinarians may also further adapt to inventory issues over time, the additional financial costs illustrated here may only be transitional.
Although the VFD has caused some financial impacts on farms, it has also promoted more involvement of veterinarians on dairy operations. The regulation has encouraged a collaboration of dairy farmers and other professionals to improve management practices to mitigate illness before treatment is needed.
So although the regulation was not preferred by most dairy farms, they do recognize the positive effect it has had on improving animal health through management methods other than antibiotic treatment. The decline in antibiotic use due to VFD has also contributed to improved antibiotic stewardship through a change in farm-level management practices. ![]()
This research was supported in part by the intramural research program of the U.S. Department of Agriculture, Economic Research Service. Kelsey O’Shea is an agriculture business management specialist with Cooperative Extension North County Regional Ag Team. Jennifer Ifft is an assistant professor and Mueller Family sesquicentennial faculty fellow in the Cornell University Charles H. Dyson School of Applied Economics and Management. Rob Lynch is a dairy herd health and management specialist with the Cornell University PRO-Dairy program.






