Infertility is the number one reason for culling on dairy operations. This is partially due to genetic selection and management for higher-producing cows, which has depressed fertility by increasing metabolic stress and blood circulation, among other mechanisms. This results in decreased estrus expression, delayed return to estrus post-calving and irregular estrus cycles, which have significant negative impacts on dairy farm expenses.
On the average-sized U.S. dairy farm of 300 cows, increasing days open from 90 to 100 days costs producers $3 per cow per day, resulting in $9,000 of increased expenses per year. Therefore, tools and technology to improve reproduction is crucial for dairy producers’ profitability. Circadian locomotor output cycles kaput (CLOCK) genes, which have long been known to regulate circadian rhythm in animals, have recently been investigated as a regulator of reproduction in dairy cattle.
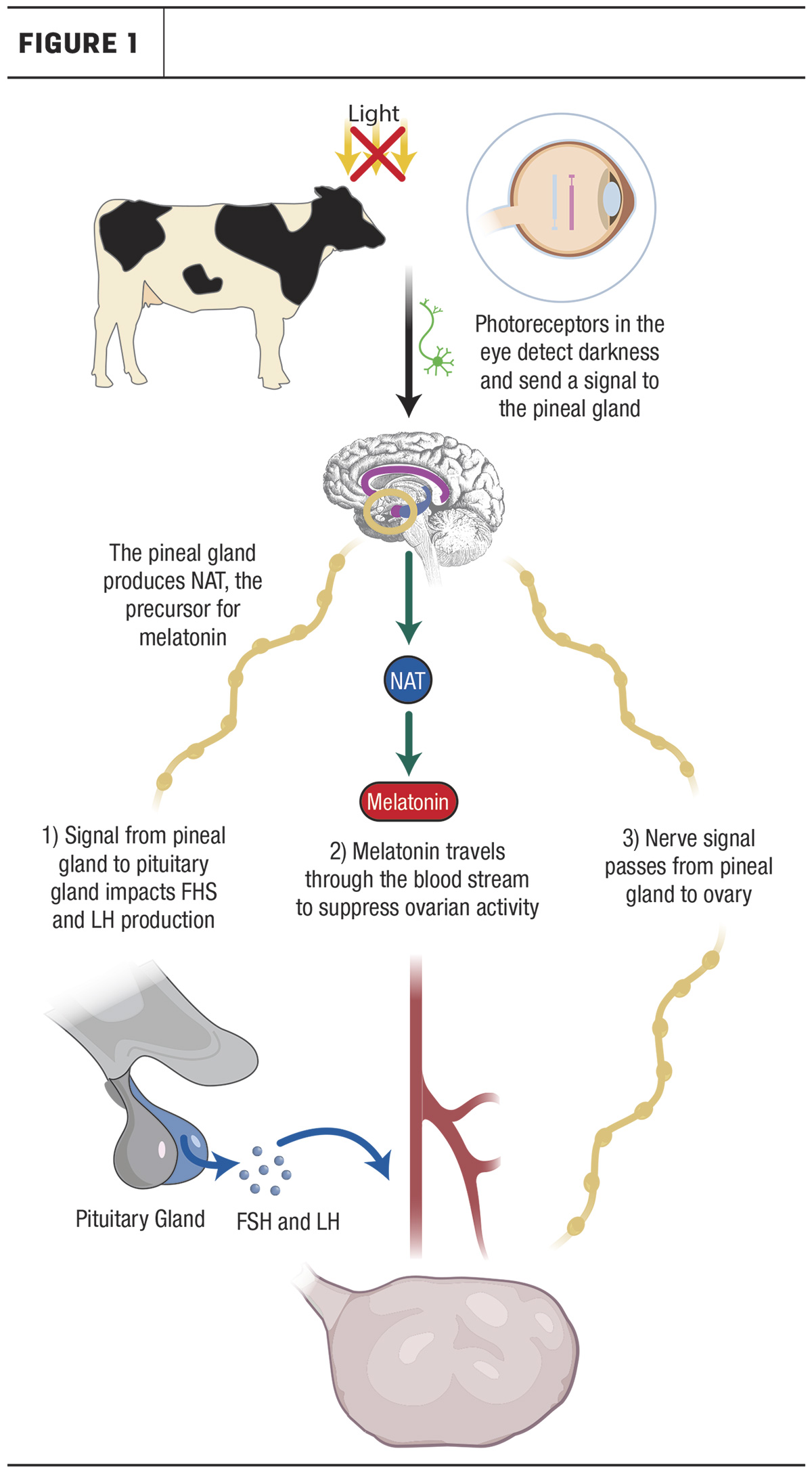
Circadian rhythm runs on a 24-hour cycle and is known as the body’s internal clock. It includes metabolic, hormonal and behavioral changes that vary within a 24-hour period. Circadian rhythm primarily responds to light and dark exposure, with one well-known example being the variation in melatonin and sleep schedule in response to light exposure. Light is detected by photoreceptors in the eye, which send a signal to the pineal gland, a small hormone-producing organ in the brain, to inhibit melatonin production. Therefore, during times of darkness, the inhibition is removed and melatonin is produced. This results in increased melatonin levels only during periods of darkness. The pineal gland serves as the overarching source of circadian rhythm regulation in animals; however, each body tissue also has a semi-independent circadian rhythm pathway that operates under the pineal gland.
CLOCK genes are a family of four core genes and proteins that aid in regulating circadian rhythm in various tissues. Expression of these genes increases during periods of darkness and causes increased production of each gene’s associated protein. These proteins then feedback negatively to cause decreased clock gene expression, in a self-regulating manner. The speed with which this feedback occurs sets the timing of the cycle, which is 24 hours in the case of most animals. Additional CLOCK genes can be found in accessory body tissues and can also impact cellular functions such as metabolism. However, little is known about how these pathways work.
Recent research shows that bovine reproductive tissues have distinct CLOCK gene expression, which seems to vary with fertility. When uterine tissue was collected and analyzed from lactating Holsteins prior to transferring embryos to them, animals that settled the embryo and maintained their pregnancy had greater expression of one CLOCK gene in their uterine tissue. Expression of this gene is increased in pregnant cattle and thought to be linked to progesterone production. This indicates a role of CLOCK genes in the uterine environment and altering uterine receptivity to embryos.
CLOCK gene expression measured from a blood sample also indicates differences in fertility. A blood sample from beef heifers that were super-stimulated for embryo collection showed that embryos collected from heifers with higher CLOCK gene expression responded better to the super-stimulation protocol. These heifers produced 11 transferable embryos on average, compared to heifers with low CLOCK gene expression, which produced only three transferable embryos. In addition, when CLOCK gene expression is removed from bovine ovarian cells in the laboratory, cell proliferation and hormone production is reduced. These findings both indicate that CLOCK genes interact at the ovarian level and regulate the ovary’s production capacity and responsiveness to hormones such as FSH, LH and estradiol.
CLOCK genes may be heritable, impacting reproductive parameters of future generations. The gene for one CLOCK gene protein has been found to have a strong association with pregnancy rate and antral follicle count in Nellore cattle. Antral follicle count, or the total number of antral follicles present on a cow’s ovary at any one time, is an indicator of an animal’s responsiveness to a super-stimulation protocol and whether several or few embryos can be recovered. Antral follicle count is also a moderately heritable trait – more heritable than most other reproductive traits discovered thus far. Therefore, the link between antral follicle count and CLOCK genes indicates potential for CLOCK genes as a genetic selection tool for fertile cows.
While the mechanism by which CLOCK genes impact reproduction is unclear, it is proposed that the pineal gland could be impacting the ovarian CLOCK in one of three pathways outlined in Figure 1: Nerve signals from the pineal gland directly stimulate the ovary; pineal gland causes FSH and LH secretion from the pituitary gland which, in turn, stimulates the ovary; and/or pineal gland production of melatonin suppresses ovarian activity. Interaction with the uterus is thought to be mainly hormonal. However, further research is needed to determine the exact role of CLOCK genes at the ovarian and uterine level.
This research indicates a role of the CLOCK family genes in reproduction and fertility in cattle with specific impacts at the ovarian and uterine level. Potential future applications could include selecting for increased reproductively efficient animals based on CLOCK gene expression or altered lighting schedules to impact reproduction favorably. While additional data is needed to fully understand how these various genes can impact reproduction, CLOCK genes show promise as another tool for explaining reproductive difficulties and improving fertility in dairy cattle.
References omitted but available on request. Click here to email an editor.





