When describing the incidence of clinical mastitis over a lactation for heifers and cows, there is a noticeable peak: a three- to sixfold increase in risk within the first seven days postpartum. We know many of these infections were acquired or maintained over the dry period.
The ultimate goal of mastitis control during the dry period is to prevent new infections from dry-off to calving as well as cure existing infections from the previous lactation.
Historical treatment and prevention
In the 1960s, to control the risk of a new intramammary infection with a contagious organism (Staphylococcus aureus or Streptococcus agalactiae), the National Institute for Research into Dairying in the UK developed the five-point plan. The five points include treating and recording clinical cases, post-milking teat dipping, blanket dry cow therapy, culling of chronic cases and milking-machine maintenance.
Modifications to this plan have been introduced, and the National Mastitis Council now proposes a 10-point plan, including the administration of an approved long-acting dry cow antibiotic into all functional quarters at dry-off plus or minus internal teat sealant.
However, control of mastitis over this period should focus not only on dry day and parlor practices but also on protecting and encouraging a cow’s natural defense mechanisms by supporting its immune system as well as providing adequate nutrition and minimizing environmental bacterial load when possible.
The immune system
In the mammary gland, normal physical barriers (such as the teat sphincter and keratin plug), cellular defenses (such as neutrophils and lymphocytes) and soluble immune factors (such as antimicrobial factors) allow a cow to clear pathogens without noticeable infection.
However, immune dysfunction postpartum results in uncontrolled inflammation and increased incidence and severity of infectious diseases. For example, while during pregnancy the dam can tolerate the growth of a fetus, respond to invading pathogens and display no clinical signs of mastitis even to highly inflammatory E. coli, in post-calving a cow can experience fever, severe clinical signs and large declines in milk production.
Such a dampened immune regulation during pregnancy is carefully biased toward T helper 2/T regulatory cell responses rather than T helper 1 and T helper 17 responses. Contributors of immune dysregulation include dramatic changes in hormone profiles as well as increased nutritional demands of lactation in the face of a negative energy balance.
Current treatment and prevention
So how do we protect and encourage natural defense mechanisms of the cow (teat-end closure) and support its immune system (innate and acquired immunity) yet prevent pathogens from appearing or persisting in the udder? A majority of cows experience delayed teat-end closure, and the keratin plug, a major barrier to microbes, is not fully formed until the process of involution is complete.
Research shows that even six weeks into the dry period, 25 percent of cows experience open/leaky teat ends. Additionally, cows that leak (oftentimes, those producing large quantities of milk at dry time) are four times more likely to experience clinical mastitis in early lactation.
Authors of a recent Journal of Dairy Science manuscript suggest abrupt dry-off followed by acute involution may cause pain and, given high levels of production, may not be best for a cow, yet methods of decreasing milk production quickly (such as feed restriction) may also be a welfare concern.
Procedures or dietary changes that produce a more gradual course of milk reduction may allow for more efficient and effective teat-end closure/keratin plug formation in the weeks following dry-off day. In addition, current data indicates teat sealant can significantly reduce the risk of new infections when used with or without antimicrobials and should be considered when developing protocols.
Next, there is a large influence of a cow’s nutritional status on immune function. For example, elevations of beta-hydroxybutyrate (ketones) during the transition period frequently lead to immunosuppression and can cause increased susceptibility to mastitis.
This is related to nutritional practices over the dry period. Subsequently, it has been shown not overfeeding cows (i.e., using controlled energy dry cow diets) in addition to providing appropriate amounts of vitamin E and selenium has metabolic and immune benefits.
We can also attempt to assist with immune response postpartum by administering several labeled products during the dry period. Coliform vaccines are commonly used to reduce clinical severity of E. coli mastitis cases postpartum. These work by stimulating the production of antibodies (IgM, IgG1 and IgG2) against antigens that gram-negative pathogens share (“common core antigens”).
Pegbovigrastim, a commercially available bovine granulocyte colony-stimulating factor, has been recently introduced to the dairy market. This product is an immunomodulator of cytokines (proteins used for crosstalk between immune cells) and, in mastitis studies, has been shown to increase the production and function of neutrophils when pathogens are present.
Last are the dry cow antimicrobial products. As scientists and producers, we have proven dry cow therapy has been instrumental in what it is labeled to do: treatment of subclinical and clinical mastitis quarters and prevention of new intramammary infections as a cow enters the dry period.
However, the verdict is still out on whether all cows need to be treated with antimicrobial therapy at dry-off in the present day.
Recent surveys in the U.S. show more than 80 percent of quarters are culture-negative at dry-off, yet the USDA-NAHMS data indicates 93 percent of cows are still treated annually in all four quarters with a dry cow antibiotic.
Selective dry cow therapy, the practice of targeting antimicrobial treatment to cows or quarters with existing intramammary infections at dry-off or those at risk for infection during the dry period, has received a large amount of attention in recent years from research, government and agricultural sectors. Why?
There are increasing global concerns over agricultural use of antimicrobial drugs, including the contribution to increased residues, possible development of antimicrobial resistance and decreased sustainability of product. Regulatory bodies in several European countries, such as Denmark and the Netherlands, are mandating the use of a selective dry cow therapy protocol, causing a reduction of dry cow antimicrobials by up to 80 percent.
An ideal selective dry cow therapy program must be easy and cost-effective to employ. It also must maximize the treatment of those cows or quarters infected while minimizing over-treatment of those cows or quarters that will likely remain healthy over the dry period.
Recent studies indicate successful candidate farms for selective dry cow therapy have bulk tank somatic cell counts of less than 250,000 cells per milliliter and few to no cows with contagious infections. The next step would be deciding what tools will be used to identify cows that should be treated, or “high-risk” cows, from those cows that will likely not benefit from treatment, or “low-risk” cows.
These tools include culture techniques that identify a cow or quarter infected, cow records that indicate mastitis cases, and monthly somatic cell counts or cow-side tests such as the California Mastitis Test. Several studies have found using several tools simultaneously to identify and not treat low-risk cows can reduce antimicrobial use without negative outcomes.
Combination criteria that include culture can be useful for farms with adequate labor, resources and the attention to detail necessary for implementation.
The authors are currently testing an algorithm that is culture-independent. The algorithm only uses programs already present on many farms, such as DC305 and DHIA tests.
The following characterizes a cow as low-risk: A somatic cell count less than 200,000 cells per milliliter at last test day before dry-off plus an average somatic cell count less than 200,000 cells per milliliter on the last three DHIA test days plus no more than one clinical mastitis event in the current lactation and no signs of mastitis at dry-off.
This computer-automated algorithm was used in a clinical trial on a New York dairy milking 1,800 cows and a bulk tank somatic cell count of 180,000 cells per milliliter.
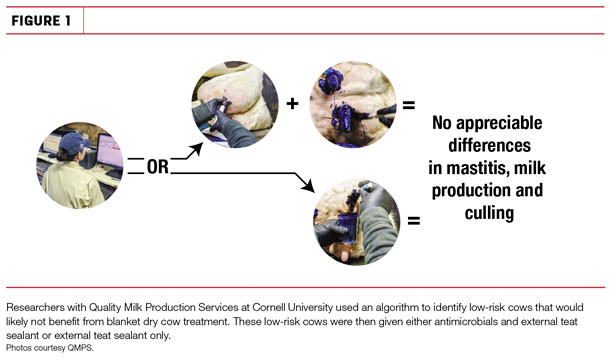
Six hundred cows identified as low-risk by the algorithm were randomly allocated to receive antimicrobials and external teat sealant or external teat sealant only. Results indicate a 64 percent allocation to the low-risk group, projecting a reduction in dry cow antimicrobial use by this percentage and a savings of approximately $6 per cow across the herd.
Preliminary analysis shows no differences in new infection risk, first-test milk production, clinical mastitis cases or culling within the first 30 days in milk when treated and untreated low-risk cows were compared (see Figure 1). Altering thresholds in any capacity within the algorithm will produce savings greater than expenditures for dry tubes when compared to blanket treating cows.
In a selective dry cow therapy program, antibiotics are still only one component of an effective mastitis control program that also includes attention to clinical cases, proper milking procedures, correctly functioning milking equipment, good udder hygiene and environment, and accurate record-keeping. Immune support via proper nutrition and perhaps vaccination and administration of immunomodulators are additional considerations. ![]()
Daryl Nydam, DVM, Ph.D., is director of quality milk production services (QMPS) at Cornell University. Amy Vasquez, DVM, is a veterinarian and Ph.D. student studying mastitis and working with the QMPS group. Email Daryl Nydam.





