Assisted reproductive techniques (ARTs) have revolutionized the field of animal breeding, as these techniques allow practitioners and scientists the ability to perpetuate the genetics of superior animals; cryopreserve genetic materials such as embryos, oocytes and sperm; allow shipping and transport of genetic material globally; enable selection and control of the sex ratio; and facilitate cloning and stem cell research.
While some ARTs are still experimental and limited in use, in vitro fertilization (IVF) and embryo transfer (ET) are increasingly used as methods to breed beef and dairy cattle because they offer the potential to produce high-merit-value offspring, achieve genetic gains and enable beef-on-dairy programs to maximize the efficiency, productivity and profitability of animal production. Due to these benefits, an estimated 2.5 million cattle embryos are transferred in the U.S. each year as cattle producers have embraced these methods to either perpetuate the genetics of their own superior donor animals, acquire new genetics through the purchase of embryos or for beef-on-dairy purposes.
IVD vs. IVP
Conventional ET, also referred to as in vivo derived (IVD) embryo transfer, allows for non-surgical collection of embryos and requires estrus synchronization and drug administration to both donor and recipient animals. During the IVF procedure, also known as in vitro produced (IVP) embryos, unovulated oocytes are aspirated from the ovaries using an ultrasound-guided transvaginal needle. The oocytes are immediately transferred to the laboratory, where they are matured, fertilized and cultured in incubators for seven days. After evaluation, the embryos can be transferred or frozen.
The total volume of both IVD and IVP embryos has increased year over year over the past two decades, but genomic testing selecting for specific genetic markers to predict the production phenotype of animals at birth has driven an increased production of IVP embryos. Despite the increased use of IVP in the dairy and beef industries, pregnancy rates of IVP typically underperform pregnancy rates observed with conventional IVD embryo transfer.
In a comparison of five independent studies evaluating pregnancy outcomes of IVD and IVP embryo transfer, IVD averaged a 55% pregnancy rate for fresh transfer and a 49% pregnancy rate for frozen transfers. In contrast, IVP demonstrated lower pregnancy outcomes averaging 42% for fresh transfer and 38% for frozen transfers.
Equally concerning is the increased embryonic loss observed in IVP embryos compared to conventional IVD embryos. In these studies, 15% of recipient animals that were confirmed for positive pregnancy at 35 days aborted the fetus and did not sustain the pregnancy to term. This is a common problem observed in the transfer of IVP embryos, likely due to the unnatural environment of the in vitro culture conditions. In a peer-reviewed article published in 2020, Peter Hansen, a professor at the University of Florida’s Institute of Food and Agricultural Sciences, describes these limitations in IVF and ET as the “incompletely fulfilled promise of embryo transfer in cattle” and states, “The key to improving pregnancy outcomes is to accurately predict embryo competence for survival and identify maternal capacity to support embryonic development.” In this article, Hansen suggests both embryonic and maternal factors are responsible for low pregnancy outcomes, and if healthy embryos and quality recipients are not selected for utilization in ET, it is unreasonable to expect the embryo to establish a pregnancy that results in a live calf.
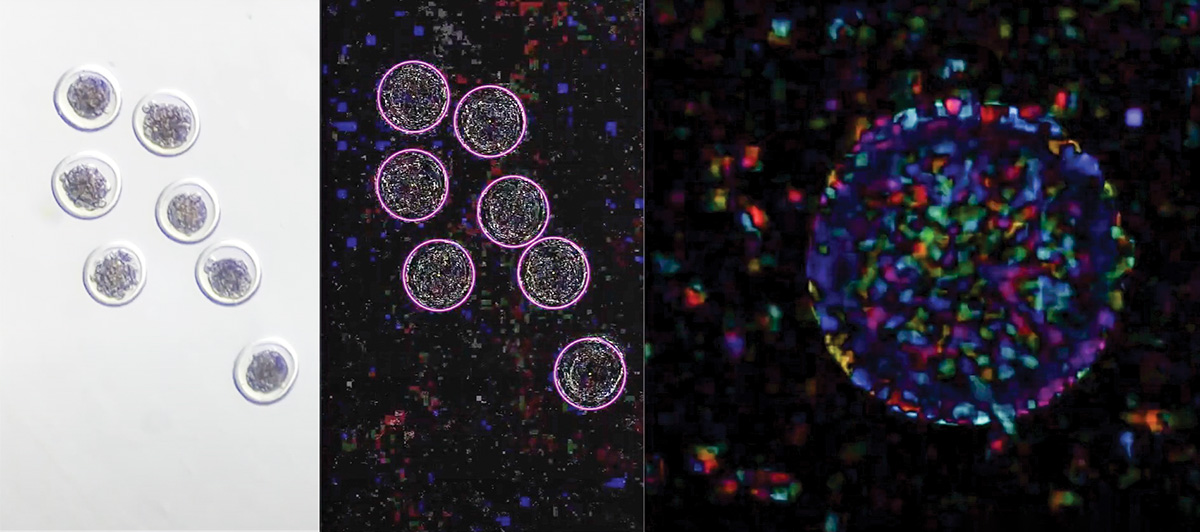
Three views of embryos offer different data points to analyze. Pictured from the left is a still shot of embryos under a microscope, a computer vision view of the same embryos and a single-embryo view of EmVision's embryo health analysis. Photo courtesy of EmGenisys.
Morphokinetics
“Morphokinetics” refers to time-specific morphological changes during embryo development providing dynamic information on a fertilized egg. The field of morphokinetics was initially driven using time-lapse monitoring systems that allow continuous, undisturbed culture of embryos and capture images of embryos at regular intervals over the span of several days. Research demonstrating the ability of embryo morphokinetics to correlate to successful embryo implantation and full-term pregnancy has been widely published evaluating human embryo quality. Due to the cost-prohibitive nature of time-lapse monitoring systems, less is known about livestock embryo morphokinetics.
To increase the practicality of embryo morphokinetic analysis for the livestock industry, a team from EmGenisys aimed to study real-time embryo morphokinetic activity of bovine embryos to reduce the observation time required to develop meaningful conclusions and eliminate the need for expensive time-lapse imaging equipment. The team hypothesized that real-time morphokinetic events could be captured on standard microscopy and camera equipment, such as a smartphone, and could reveal critical indicators of embryo viability. It is well documented that growing embryos are rapidly undergoing metabolic and mitotic processes, as they must develop from a one-cell zygote to a fully functioning calf in the span of 285 days. Capture and analysis of this metabolic and mitotic activity could provide objective data to evaluate embryo health and allow for a more standardized embryo evaluation process.
In a pilot study published in the American Embryo Transfer Association newsletter in 2021, 94 IVD embryos were collected from beef cattle. A 35-second video of each embryo was recorded with a camera mounted in a trinocular stereo zoom scope. To study the embryos’ morphokinetic changes during short, 35-second observation periods, area, diameter and subzonal space were measured at five-second intervals in amplified videos. Comparisons were made between embryos in each group (embryos videoed together) to determine whether morphokinetic changes were unique and independent to the embryo (rather than artifacts inducing noise in the video data) and between embryos that did and didn’t establish pregnancies.
This data showed that embryos recorded in the same video demonstrated low correlation, suggesting embryos in a single video file demonstrated unique, individual growth trends. Pregnancy results from this study suggest embryos with moderate morphokinetic activity establish pregnancies at a greater rate than embryos with a high or low level of morphokinetic activity. Overall, this field study demonstrated that embryo morphokinetic changes can be measured in short observatory periods and can be used to build mathematical models correlating to an embryo’s developmental potential, competency and viability to provide a more comprehensive evaluation of an embryo’s health, spanning beyond a morphological analysis.
Machine learning and future technology
Machine learning is a type of artificial intelligence that can learn and adapt without being explicitly programmed by using algorithms and statistical models to analyze and draw inferences from labeled data. In simple terms, we can “teach” machine learning technology to recognize patterns associated with images or videos of either viable or non-viable embryos, so the model can predict embryo viability when it scans a new image of an embryo when viability outcomes are unknown.
New methods to objectively evaluate embryo health can unlock the potential of ET and IVF services to improve animal health, production economics and environmental sustainability. By leveraging advancements in machine learning and imaging technologies, new objective methods can be created to evaluate embryo health, which will allow ET practitioners, embryologists and producers methods to better manage their breeding strategies. Further research is in progress to improve prediction accuracy of machine learning technologies to evaluate embryo viability, predict embryo sex, detect subclinical signs of embryo stress, detect early embryonic loss, detect large offspring syndrome and other clinically relevant characteristics of embryos that result in economic loss or detriment to cattle producers. Additionally, studies to develop machine learning models to evaluate embryo health of other species, including small ruminants and horses, are in progress.









