Quickly and accurately characterizing the nutrient content of feedstuffs is vital for balancing high-performance dairy cow rations. It also allows manufacturers to develop robust quality control programs and represent their products as precisely as possible. When it comes to supporting overall animal health and performance, one of the most important nutrients we evaluate as an industry is protein.
High-protein feed ingredients like blood meal and bypass soy products play a pivotal role in providing rumen-undegradable protein (RUP) in rations. However, these feeds can vary widely in quality due to processing differences. This makes lab analysis critical for evaluating their relative merits. For high-protein feeds, there are three metrics that are particularly useful in quantifying ingredient quality: crude protein (CP), RUP and intestinal digestibility. The amino acid profile of these feeds is also important, but at this time it is generally not economically feasible to run individual amino acid analysis on a routine basis.
Protein analysis over the decades
Crude protein was first characterized in 1865 at an experimental research station in Germany and is the simplest estimation of protein quality. It is measured by multiplying feed nitrogen by 6.25 (assuming feed protein is 16% nitrogen on average; 1 / 0.16 = 6.25). While greater emphasis is now put on metabolizable protein (MP) and amino acids when evaluating rations, crude protein still serves as the foundation for more advanced estimates of protein quality and calculations in ration software models.
About 100 years after crude protein made its debut, labs began estimating rumen and intestinal degradation of feed nutrients (primarily fiber). Over the subsequent 40 years, this concept was applied to protein with several additions and modifications made to the basic protocol, resulting in what is known today as the Three-Step method. This involves both an in situ (in cow) portion and an in vitro (in a test tube) portion. The in situ portion involves incubating feed samples in sturdy, porous bags suspended in the rumen of cannulated cows for 16 hours to estimate RUP/RDP. The resulting residue is then further incubated in vitro with digestive enzymes, such as pepsin and pancreatin, to estimate intestinal digestibility.
In 2013, researchers at Cornell University introduced a new method for analyzing protein quality. Known as the Ross, Cornell or Multi-step in vitro protein evaluation (MSPE) method, this fully in vitro protocol was designed to help increase repeatability and improve upon other limitations of the previous method.
While both methods have their benefits, they also have limitations. The Three-Step’s use of bags incubated in the rumen can allow for the loss of small particles through the pores in the bag or for excessive microbial protein retention in the bag, which can skew RUP estimates lower or higher, respectively. Washout corrections can be used to try and account for the loss of small particles. Additionally, different versions of the Three-Step method use different digestive enzymes, which affects the intestinal digestibility estimate and makes it difficult to compare results between labs.
Conversely, the Cornell method was originally designed to only provide an undigestible protein number for use in the Cornell Net Carbohydrate and Protein System (CNCPS) ration model and was later adapted to provide a RUP value. These RUP estimates should be interpreted with caution. As with any newer method, the Cornell method has not been validated against in vitro data in as many situations or with as many feeds. Since both assays use rumen fluid, they are also both subject to animal as well as lab-to-lab variation. Commercial analytical labs try to mitigate these effects by running standard check samples and multiple subsamples of the same feedstuff.
Evaluating currently available methods
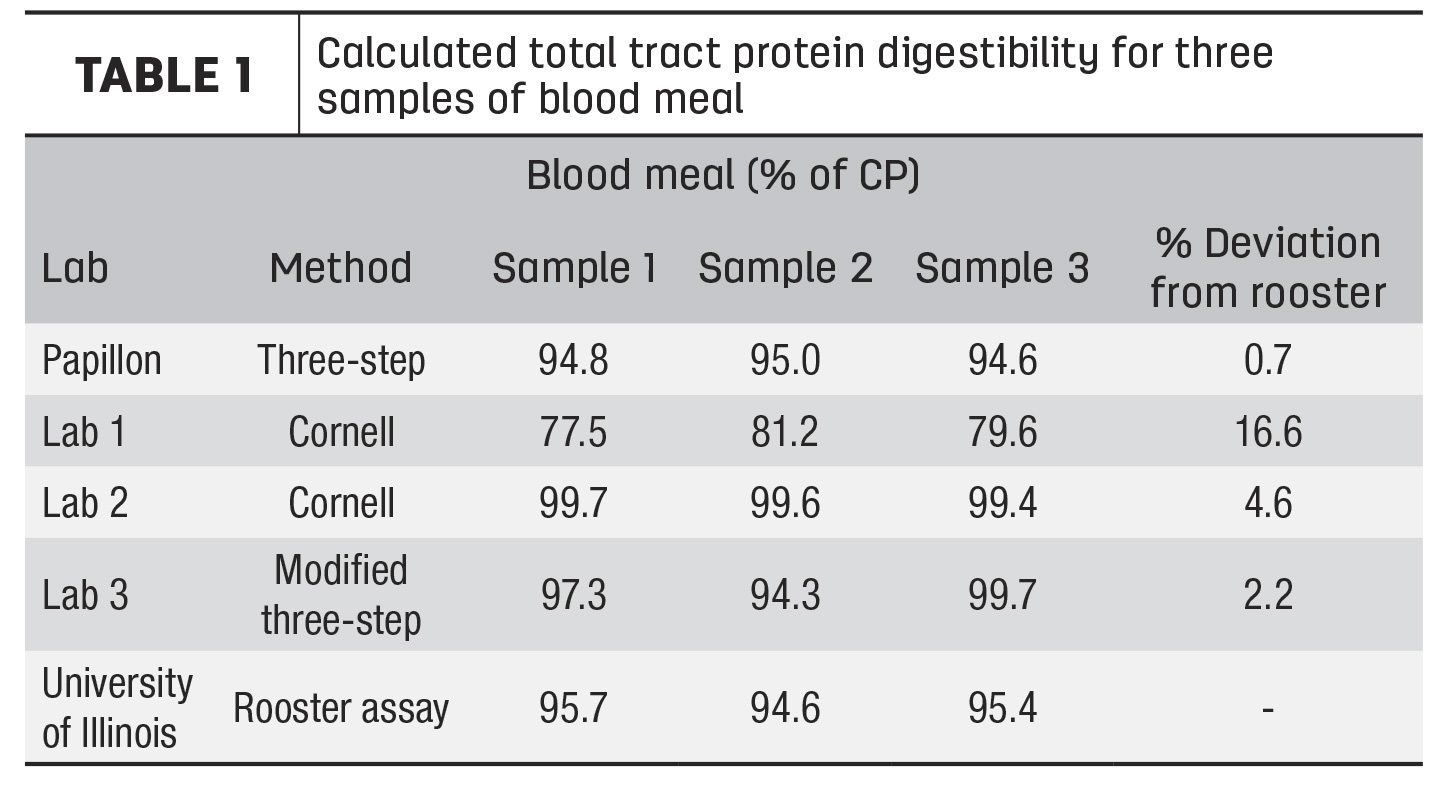
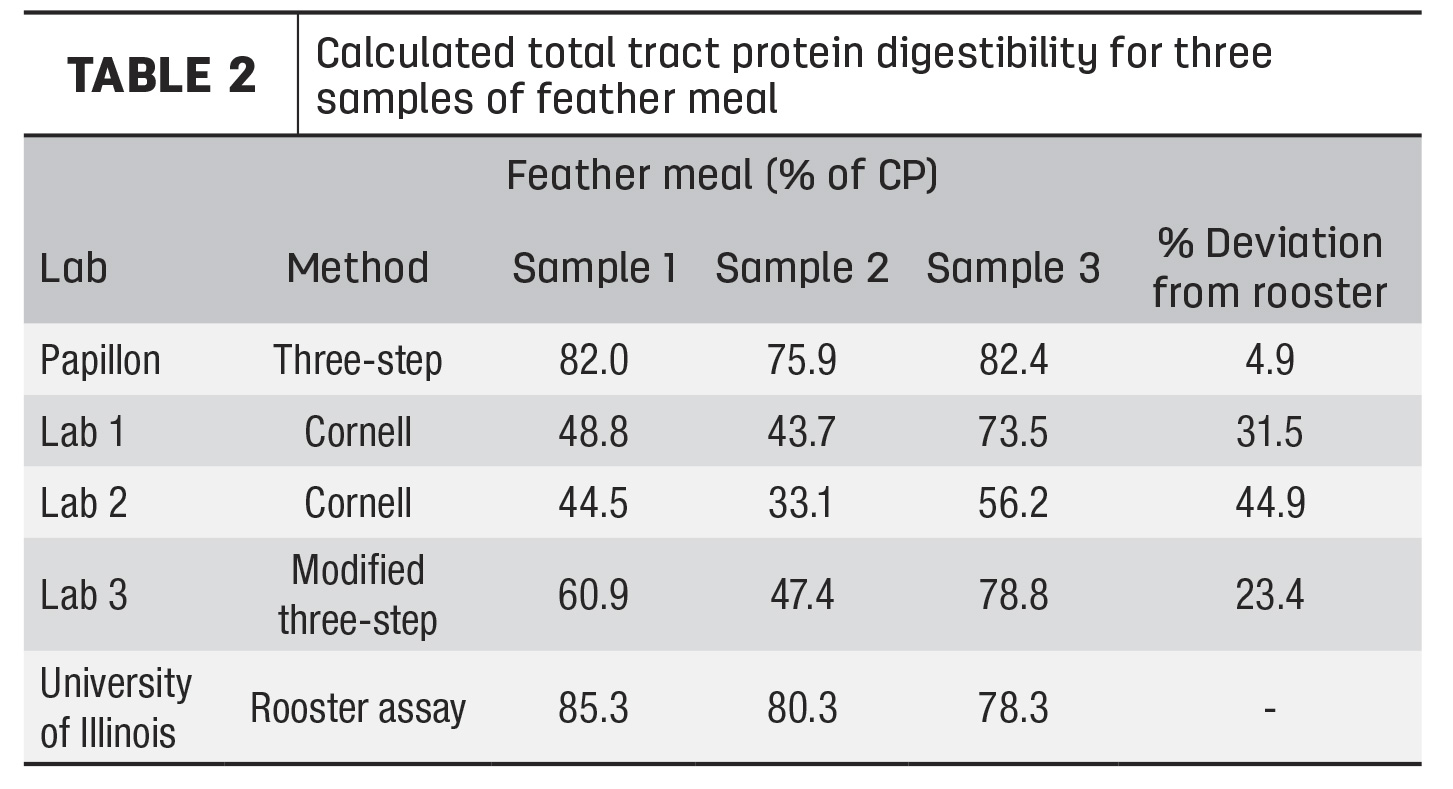
Our team conducted an experiment to assess the commercially available methods for evaluating RUP and intestinal digestibility of animal-rendered byproducts. Three samples of both high-quality blood meal and feather meal were collected from three different semi-loads of product from the same supplier. Subsamples of each were sent to two commercial forage analysis laboratories offering the Cornell method and to one commercial forage analysis laboratory running the modified Three-Step method.
Samples were also run through our company’s traditional Three-Step method and through the cecectomized rooster bioassay at the University of Illinois. The cecectomized rooster assay is a biological assay where roosters are fasted for 24 to 48 hours and then fed a specific amount of the ingredient of interest. The excreted protein is measured and used to determine total tract digestibility. This assay was chosen because it is considered the gold standard for completely in situ estimates of digestibility.
The results for blood meal and feather meal can be seen in Tables 1 and 2, respectively. These results are presented as total tract digestibility, which accounts for both RUP and intestinal digestibility measurements. Across all samples, the blood meal results were very similar to the rooster bioassay, with less than 5% deviation for most analysis methods, except for one lab, which has since improved their method. For feather meal, total tract digestibility of all samples except for the traditional Three-Step were variable both between and within labs and were on average much lower than the rooster assay. The traditional Three-Step was the only analysis that was within 5% deviation from the rooster bioassay. Feather meal is a keratin protein and differs from most vegetable and other animal proteins, such as blood meal, in that it is high in the structural amino acids cysteine and proline. These differences result in a protein structure that is much more difficult to characterize than simpler feeds.
At the writing of this article, all three major U.S. labs offer the Cornell assay, and two of the three offer some form of the Three-Step. The differences between offered assays and protocols between labs can introduce some confusion when it comes to interpreting protein analysis results, especially if they were run at different labs with different methods. As with most feed analysis, the best way to overcome these challenges is to become familiar with an analysis and results from one lab and stick with that lab using previous analysis to benchmark future analysis.







